I2U System Readiness Tool
Purpose of The Tool
This Tool is not intended to replace health technology assessments conducted by Canada’s Drug Agency (CDA-AMC), l’Institut national d’excellence en santé et en services sociaux (INESSS) and other agencies or organizations.
Instead, the tool complements and supplements foundational HTA efforts by helping health system leaders answer three fundamental questions at the heart of implementation excellence:
WHAT
key implementation enablers are most relevant for the successful launch of a breakthrough therapy?
HOW
ready are Canadian health systems to launch a given breakthrough therapy – based on the implementation enablers outlined above?
WHAT
measures can policymakers and payers take today to strengthen those key implementation enablers and “future-proof” their health systems?
By working through the tool, our hope is that health system leaders, patient group representatives and innovators will find the insights, analysis, and recommendations required to jump-start essential conversations so that they occur months or even years prior to implementation.
Overview of The Tool
Section 1: Implementation Enablers Framework
Developed by I2U, this Framework is a theoretical foundation that captures the wide range of “implementation enablers” that could support the effective system implementation of a breakthrough therapy.
1. INFRASTRUCTURE | 2. HEALTH HUMAN RESOURCES | 3. POLICIES & PROTOCOLS | 4. MONITORING & EVALUATION |
|---|---|---|---|
1.1. Manufacturing (e.g., cleanrooms, bioprocessing facilities, device manufacturing facilities, pharmaceutical manufacturing plants, packaging and labeling facilities, quality control and testing labs, warehouse and distribution centre, R&D labs) | 2.1. Nurses (e.g., registered nurses, registered practical nurses, and nurse practitioners) | 3.1. Scopes of Practice | 4.1. Data Collection and Analysis |
1.2 Cold Chain (e.g., cold storage units, temperature monitoring devices, cold chain packaging, temperature-controlled transportation vehicles, cold chain distribution centre, cold chain management software, emergency back-up cold chain systems) | 2.2. Primary Care Providers (e.g., Family Physicians) | 3.2. Eligibility & Referral Protocols | 4.2. Knowledge Translation |
1.3. Imaging (e.g., CT, PET, MRI, X-Ray) | 2.3. Specialist Physicians (e.g., Internal Medicine physicians, Pediatricians, Geriatricians, Neurologists, Cardiologists, Dermatologists) | 3.3. Health Care Provider Guidelines | |
1.4. Nuclear Medicine (e.g., cyclotrons, lead-lined rooms, radiation safety PPE, gamma cameras, radiopharmacy, radioisotope production facilities) | 2.4. Pharmacists | 3.4. Education & Training | |
1.5. Diagnostic Labs (e.g., hematology labs, microbiology labs, clinical chemistry labs, molecular diagnostics labs, genomics testing labs, pathology labs, toxicology labs, cytology and histopathology labs, clinical immunology labs) | 2.5. Technicians (e.g., medical lab technicians, electrocardiogram technician, radiologist technologist) | 3.5. Regional and or Geographic Dynamics (e.g., Centres of Excellence models, requirements for out-of- province care delivery, measures to ensure equitable access across urban/rural settings etc.) | |
1.6. Physical Space (e.g., patient rooms, surgical suites, diagnostic areas, staff areas, equipment rooms, storage) | 2.6. Administration (e.g., medical receptionists, patient services coordinator, health care compliance officer) | 3.6 Equity and Diversity Policies (e.g., measures to ensure equity of access across the population, including gender, language, Indigenous, race and ethnicity considerations) | |
1.7. Specialized and General Delivery Equipment (e.g., infusion machinery, dialysis machinery, oxygen, water filtration, wheelchair) | 2.7. Personal Support Workers | ||
1.8. Computer and IT Systems (e.g., scheduling software, electronic health records, virtual care / remote monitoring platforms, patient portals, communications platform, laboratory information systems) | 2.8. Other Allied Health Professionals (e.g., respiratory therapist, physical therapist, occupational therapists, nutritionists, speech language therapists) |
Section 2: Relevance Assessment
The Relevance Assessment will guide the user to answer the first fundamental question: WHAT key implementation enablers are most relevant for the successful launch of a breakthrough therapy?
Section 3: Readiness Assessment and Settings Selection
The Readiness Assessment will guide the user to answer the second fundamental question: HOW ready are Canadian health systems to launch a given breakthrough therapy – based on the implementation enablers outlined above?
Additionally, there is a Settings Selection question for each Implementation Enabler identified as “Relevant”. Coupled with the prioritization exercise (next section), the Settings Selection exercise is designed to help system leaders determine WHERE to focus their time and resources and WHO needs to come together to begin developing solutions to reduce or remove significant implementation barriers.
Section 4: Prioritization of Relevant Enablers
This section of the Tool provides an opportunity for users to prioritize enablers that are critical for implementation. The prioritization exercise goes beyond identifying relevant implementation enablers that exist within a system. By narrowing in on the factors that require immediate attention, it provides a stepping stone to identify solutions - helping users determine where to focus efforts, budget and planning.
To access the Tool, please click the link below and create an account free of charge. By creating an account, your work can be saved and revisited later.
In the future, I2U will be providing some additional consultation services that can be layered to provide more in-depth analysis and recommendations.
FAQs
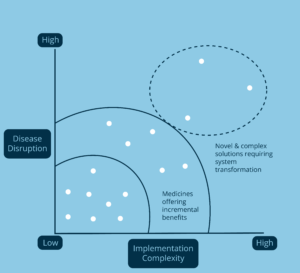
As shown in Figure 1, Breakthrough Therapies are those therapies that combine (1) the greatest improvement vs. the current standard of care; and (2) the most complex challenges impeding the uptake required to maximize patient and system value.
There are no formal prerequisites for using the Tool.
Having robust and comprehensive information and knowledge on the breakthrough therapy in question, including its indications, production needs, requirements for administration, will be helpful when going through the Tool.
Yes, the Tool was designed to assess a wide variety of breakthrough therapies, from gene therapy to radionuclide therapy to other disease-modifying therapies.
If you experience any technical issues, or have any questions, comments, or concerns, please reach out to info@I2U.ca.
